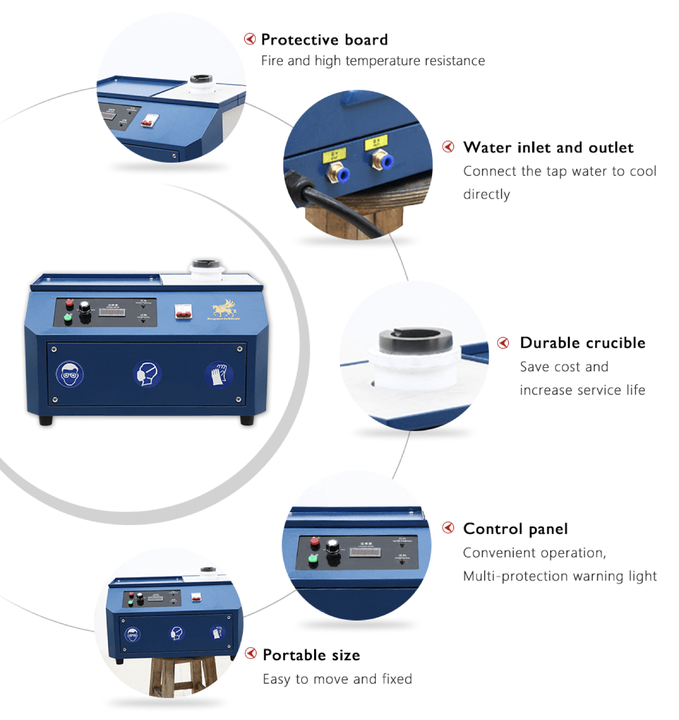
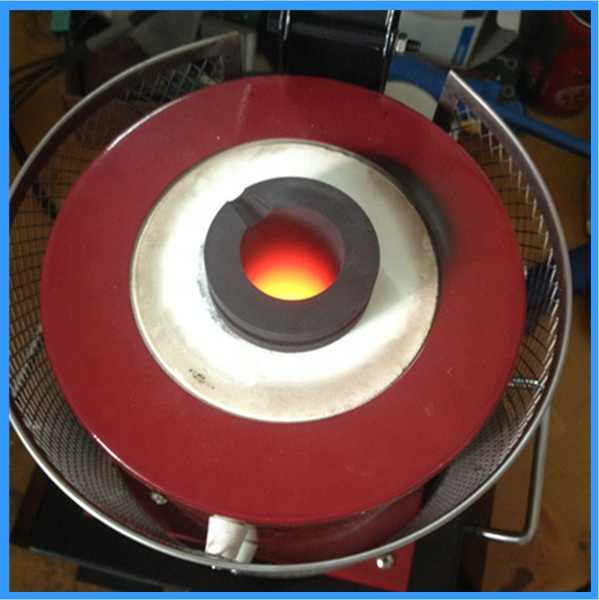
nickel and silver smelting

Nickel and Silver Smelting: A Comprehensive Guide
Smelting is an ancient process used to extract metals from their ores, and it plays a vital role in both industrial and artisanal metalwork. Nickel and silver are two metals often refined through smelting, though their processes differ slightly. Understanding how nickel and silver smelting works is crucial for industries involved in metal production, as well as for small-scale operations interested in refining metals for various applications.

What is Smelting?
Smelting involves heating ores to high temperatures to extract pure metals from the surrounding rock or other materials. In the case of nickel and silver, this means separating these valuable metals from other elements and compounds in their respective ores or scrap. The process requires specialized equipment and chemical reagents to remove impurities and refine the metal.
Smelting Nickel: Process Overview
Nickel is commonly found in ores such as pentlandite and laterites. Smelting nickel involves multiple steps, as nickel ores are often bound with sulfur and iron. The primary smelting method for nickel is pyrometallurgy, which uses heat and chemical reactions to extract the metal.
- Ore Concentration
- The first step in nickel smelting is to concentrate the ore by crushing and grinding it into smaller particles. Flotation methods are used to separate the nickel from the waste material, leaving behind a concentrated nickel-rich mixture.
- Roasting and Smelting
- Once concentrated, the nickel ore is roasted in a furnace to remove sulfur. The next stage is smelting, where the roasted ore is mixed with fluxes like limestone or silica and heated to over 1,500°C. The molten metal sinks to the bottom, and slag (impurities) rises to the top, which is then removed.
- Converting and Refining
- The smelted nickel is then converted into purer forms by blowing oxygen through the molten metal. This step removes any remaining sulfur and iron. Electrolytic refining is often used to produce high-purity nickel.
- Advantages of Nickel Smelting:
- Produces high-grade nickel used in stainless steel and alloys.
- Can process large quantities of ore efficiently.
- Challenges:
- Requires high temperatures and specialized equipment.
- Nickel smelting produces significant amounts of sulfur dioxide, which must be captured to reduce environmental impact.

Smelting Silver: Process Overview
Silver smelting, on the other hand, is a more straightforward process compared to nickel due to silver’s lower melting point and fewer chemical complexities in its ores. Silver is typically extracted from argentiferous ores or recycled scrap through smelting.
- Preparation of Ore or Scrap
- The silver-bearing ore or scrap is crushed and prepared for smelting. In the case of ore, flotation or gravity methods are often used to concentrate the silver.
- Flux Addition
- Fluxes like borax, soda ash, or silica are added to the furnace. These fluxes help to separate the silver from impurities by forming slag, which can be easily removed from the molten silver.
- Melting and Smelting
- The ore or scrap is placed in a crucible or furnace, and heat is applied. Silver melts at around 961°C, and once it becomes molten, the impurities rise to the surface as slag, which is skimmed off. The remaining metal is high-purity silver.
- Pouring and Cooling
- The molten silver is poured into molds, allowed to cool, and solidify into bars or ingots. The refined silver is ready for use in jewelry, electronics, and industrial applications.
- Advantages of Silver Smelting:
- Produces high-purity silver (up to 99.9%).
- Can be done on both large and small scales.
- Challenges:
- Requires careful control of temperature and flux materials to achieve high purity.
- Silver smelting, especially with ores, can produce toxic fumes.
Differences Between Nickel and Silver Smelting
While both nickel and silver are refined using smelting, the processes have key differences due to the properties of each metal and the composition of their ores.
- Melting Points: Nickel melts at a much higher temperature (1,455°C) than silver (961°C). As a result, nickel smelting requires more energy and advanced furnace technology.
- Chemical Complexities: Nickel ores are often combined with sulfur and iron, which requires a multi-stage process, including roasting, smelting, and refining. Silver ores are typically simpler to process, with fewer stages required to extract the metal.
- Environmental Impact: Nickel smelting has a higher environmental footprint due to the release of sulfur dioxide gas, which must be managed to prevent acid rain. Silver smelting is generally cleaner, though the use of fluxes and potential for releasing harmful fumes must still be monitored.
Modern Advances in Nickel and Silver Smelting
Recent advances in smelting technologies have made both nickel and silver smelting more efficient and environmentally friendly. Some of these advancements include:
- Flash Smelting for Nickel: This technology uses high-intensity heat and oxygen to reduce energy consumption and emissions. It is widely used in modern nickel smelting plants.
- Microwave Smelting for Silver: Microwave smelting is an emerging technology that allows for small-scale silver smelting with less energy and fewer emissions compared to traditional methods.
- Recycling and Scrap Smelting: As industries push towards sustainability, smelting processes for recycled nickel and silver have become more refined, reducing the need for mining and lowering environmental impacts.
Nickel and silver smelting are essential processes in the metal refining industry, each with its unique challenges and advantages. While nickel smelting involves higher temperatures and more complex chemical reactions, silver smelting is more straightforward but still requires careful attention to detail. With ongoing technological advancements, both metals can be refined efficiently and sustainably, meeting the growing demand for pure nickel and silver in various industries.