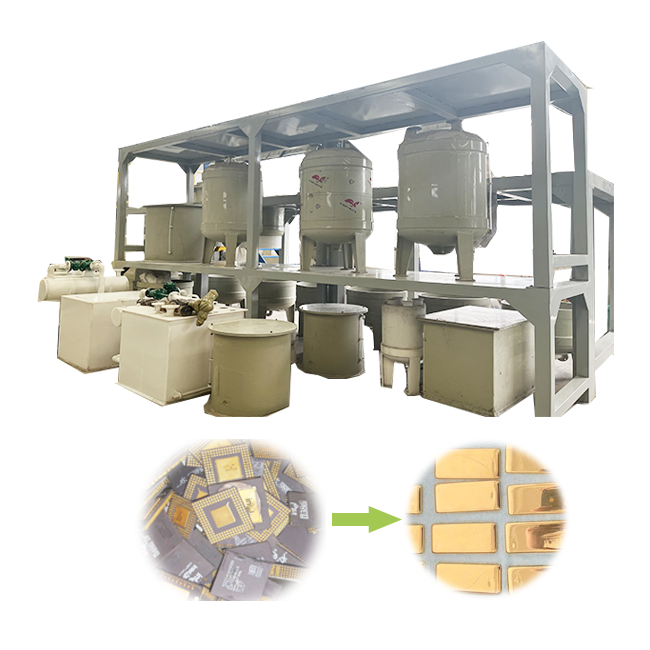
nitric acid gold refining






Nitric Acid Gold Refining: A Comprehensive Guide
Nitric acid gold refining is one of the most common and efficient methods for purifying gold. This technique has been widely used for centuries, and today, it remains a valuable method for small-scale refiners and professional operations alike. The process of refining gold using nitric acid involves the removal of base metals, leaving behind pure gold. In this article, we will explore the nitric acid gold refining process, its benefits, and safety precautions.
Understanding Nitric Acid Gold Refining
Nitric acid gold refining is a chemical process that removes impurities from gold by dissolving base metals such as silver, copper, and zinc. Nitric acid itself does not dissolve gold, which makes it an ideal medium for separating gold from other metals. By selectively dissolving base metals, nitric acid helps isolate gold in its purest form.
The Chemistry Behind Nitric Acid Gold Refining
The chemical reaction involved in nitric acid gold refining is straightforward. When base metals are exposed to nitric acid, they react to form soluble nitrates, which can be easily removed from the gold. The reaction occurs as follows:
- Nitric acid (HNO₃) reacts with base metals such as copper or silver.
- The base metals dissolve in the acid, forming metal nitrates.
- Gold, being inert to nitric acid, remains intact as a solid residue.
This selective dissolution allows for the purification of gold without damaging the precious metal itself.
Steps in Nitric Acid Gold Refining
Nitric acid gold refining involves several steps, each of which requires careful attention to ensure a successful purification process. Below is a general outline of the process:
Preparing the Gold Material
Before beginning the refining process, the gold material must be prepared. The gold is usually in the form of scrap, old jewelry, or gold-containing materials. Any base metals mixed with the gold need to be removed using nitric acid. Ensure that the gold is clean and free of any debris.
Dissolving Base Metals
The next step is to dissolve the base metals from the gold. To do this, the gold-bearing material is placed in a container, and nitric acid is added. The acid reacts with the base metals, dissolving them into the solution while leaving the gold unaffected.
- Add the nitric acid slowly to avoid splashing or excessive bubbling.
- Allow the reaction to continue until all the base metals have dissolved.
After the dissolution of the base metals, what remains in the container is gold, which is now ready for further purification.
Removing Impurities
Once the base metals have been dissolved, the solution needs to be filtered to remove any solid impurities. The gold, which remains as a solid, is separated from the liquid solution containing dissolved nitrates. Specialized filtering materials can help ensure a clean separation of gold from the liquid.
- Pour the solution through a filter to capture the solid gold particles.
- Discard the liquid solution safely, following all environmental regulations.
Washing and Drying the Gold
After filtering out the gold, the next step is to wash it thoroughly to remove any remaining traces of acid or impurities. This ensures that the final product is free of contaminants.
- Rinse the gold with distilled water to remove any leftover acid.
- Allow the gold to dry completely before melting or further processing.
Once dried, the gold can be melted and cast into bars or other forms, depending on the desired end product.
Advantages of Nitric Acid Gold Refining
Nitric acid gold refining offers several key advantages over other methods. These benefits make it a popular choice for both small-scale hobbyists and professional refiners.
High Purity Gold
One of the main advantages of nitric acid gold refining is the ability to achieve high-purity gold. By selectively dissolving base metals, the process leaves behind gold that is often 99.9% pure.
Cost-Effective Method
Nitric acid gold refining is relatively inexpensive compared to other refining methods. The chemicals required, such as nitric acid, are widely available and affordable, making this method accessible to those on a budget.
Simple and Efficient
The refining process itself is straightforward and can be completed with minimal equipment. This simplicity makes it an attractive option for small-scale refiners looking to purify gold from scrap material.
Safety Precautions When Refining Gold with Nitric Acid
While nitric acid gold refining is an effective method, it also involves handling hazardous chemicals. Refiners must take appropriate safety precautions to prevent accidents or exposure to harmful substances.
Proper Ventilation
Nitric acid produces toxic fumes during the refining process, so it is essential to work in a well-ventilated area. If possible, use a fume hood or conduct the process outdoors to minimize exposure to harmful gases.
Protective Gear
Always wear appropriate protective gear when working with nitric acid. This includes gloves, goggles, and a chemical-resistant apron. Nitric acid can cause severe burns if it comes into contact with skin, so it is crucial to avoid direct exposure.
Safe Disposal of Waste
The waste produced during nitric acid gold refining must be disposed of safely. Do not pour leftover acid or nitrates down the drain. Instead, follow local regulations for the disposal of hazardous waste to ensure that you are protecting the environment.
Challenges of Nitric Acid Gold Refining
While nitric acid gold refining is a powerful method, it does come with some challenges that refiners need to be aware of.
Not Effective for Alloys with Platinum or Palladium
Nitric acid gold refining is highly effective for removing base metals, but it is not suitable for alloys containing platinum or palladium. These metals are also resistant to nitric acid, so other refining methods must be used to separate them from gold.
Requires Careful Chemical Handling
Handling nitric acid and other chemicals used in the refining process requires knowledge and caution. Beginners may find the process intimidating due to the risks associated with working with corrosive acids.
Nitric acid gold refining is an efficient and cost-effective method for purifying gold from base metals and impurities. By selectively dissolving other metals, nitric acid helps isolate high-purity gold, making it a valuable process for small-scale refiners and professionals alike. However, it is essential to follow all safety guidelines when working with nitric acid and to dispose of waste responsibly. With the right equipment and precautions, nitric acid gold refining can be a rewarding and effective way to achieve pure gold.