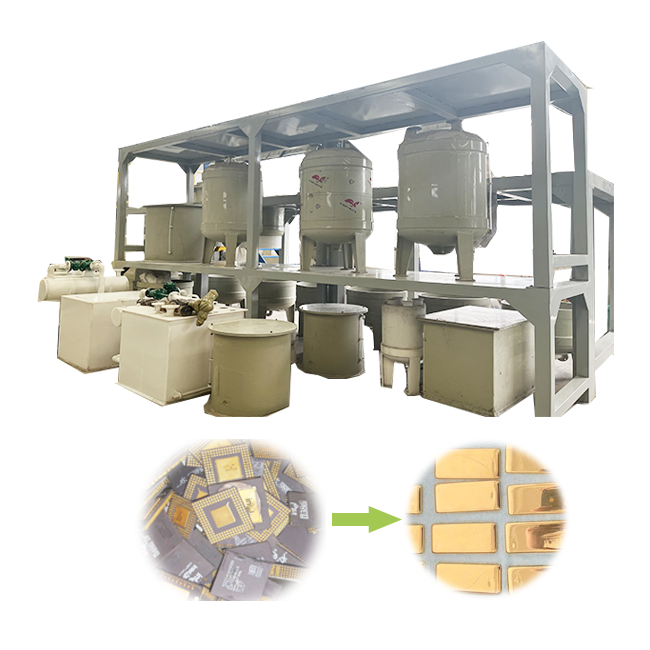
refining gold with electrolysis


Refining Gold with Electrolysis: A Modern Approach to Purity
Refining gold with electrolysis is one of the most efficient and precise methods for purifying gold. This process uses electrical currents to separate impurities from gold, leaving behind high-purity metal. Compared to traditional methods such as smelting, electrolysis offers greater precision and can achieve up to 99.99% purity. In this article, we will explore the electrolysis process for refining gold, its advantages, and how it works.
What Is Electrolysis?
Electrolysis is a chemical process that uses electricity to break down compounds into their constituent elements. When applied to gold refining, electrolysis involves passing an electric current through a gold solution, which causes the gold to migrate to a cathode while impurities are left behind. The result is highly refined gold.

Why Use Electrolysis for Refining Gold?
The process of refining gold with electrolysis has several advantages over other methods:
- High Purity: Electrolysis can achieve gold purity levels of up to 99.99%, making it ideal for industrial applications such as electronics and jewelry production.
- Efficiency: The process is highly efficient, allowing for the extraction of gold from solutions with minimal waste.
- Precision: Electrolysis offers precise control over the refining process, ensuring consistent and high-quality results.
- Environmentally Friendly: Unlike some traditional methods, electrolysis does not produce harmful emissions or toxic byproducts, making it a cleaner and more sustainable option.
How Does Gold Refining with Electrolysis Work?
The process of refining gold using electrolysis involves several key steps, which we will break down below:
Step 1: Preparing the Gold Solution
The first step is to dissolve the gold into a solution. This is typically done using a combination of chemicals, such as a mixture of hydrochloric acid (HCl) and nitric acid (HNO₃), known as aqua regia. The acids break down the gold into a liquid form, which can then be used in the electrolysis process.
Step 2: Setting Up the Electrolytic Cell
The gold solution is placed in an electrolytic cell, which contains a cathode and an anode. The cathode is made of a highly conductive material, often stainless steel or copper, where pure gold will be deposited. The anode is typically a piece of impure gold, from which the gold is dissolved into the solution.
Step 3: Applying Electrical Current
An electric current is passed through the solution, causing the gold ions in the solution to migrate toward the cathode, where they are deposited as pure gold. As the current continues, more gold is separated from the impurities, which remain in the solution or fall off the anode as an insoluble byproduct.
Step 4: Collecting the Refined Gold
Over time, a layer of pure gold will build up on the cathode. Once enough gold has been collected, the cathode is removed, and the gold is scraped off. This gold is then melted and cast into bars or other desired forms. The remaining solution and impurities can be processed further, ensuring that no gold is wasted.

Benefits of Refining Gold with Electrolysis
There are several notable benefits to using electrolysis for refining gold:
- Superior Purity: Electrolysis can produce gold of the highest purity, making it suitable for high-end applications.
- Lower Environmental Impact: This method produces fewer emissions and toxic byproducts compared to other refining techniques.
- Cost-Effective: Once the initial setup is in place, electrolysis can be a cost-efficient method for gold refining, especially when refining large quantities of metal.
- Minimal Waste: The process minimizes waste and ensures that most of the gold is recovered from the initial solution.
Applications of Electrolytic Gold Refining
The ability to produce high-purity gold through electrolysis makes it a valuable method in several industries:
- Electronics: High-purity gold is essential in the production of electronic components such as connectors and circuit boards due to its excellent conductivity.
- Jewelry: Jewelers require refined gold to ensure that their products are of the highest quality.
- Dentistry: Dental work often uses high-purity gold for crowns and fillings due to its biocompatibility and durability.
Refining gold with electrolysis is a precise and efficient method for obtaining high-purity gold. By using electric currents to separate gold from impurities, this technique ensures that industries requiring the purest gold can obtain it in an environmentally friendly manner. The benefits of the process, including its efficiency, minimal waste, and high precision, make it an excellent option for refining gold at an industrial scale. As technology continues to advance, electrolysis is likely to remain a cornerstone of modern gold refining processes.