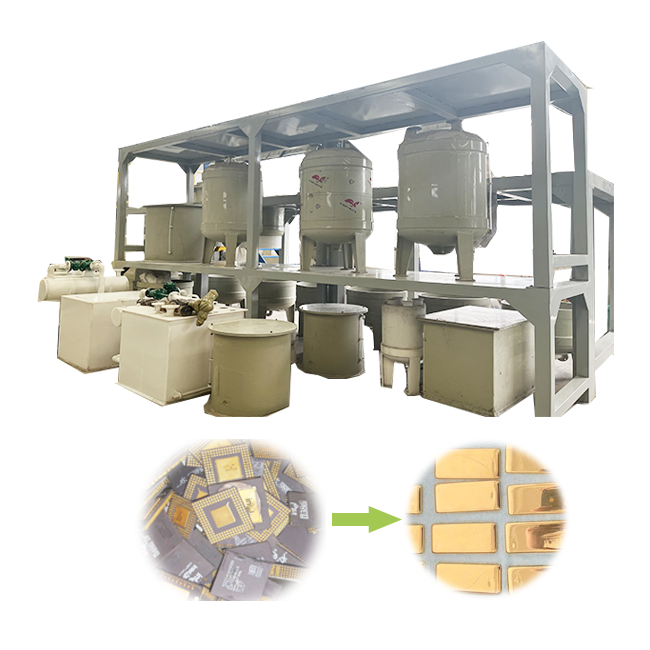
refining gold with nitric acid

Gold refining is an essential process for transforming raw gold into its purest form. One of the most effective methods for this task is refining gold with nitric acid. This technique is renowned for its precision and effectiveness in separating gold from other metals. In this article, we will explore the process of refining gold with nitric acid, highlighting its methods, benefits, and considerations.


The Process of Refining Gold with Nitric Acid
Refining gold with nitric acid involves a specific set of steps designed to purify gold from alloys or ores. The process starts with dissolving the gold material in aqua regia, a potent mixture of nitric acid and hydrochloric acid. Aqua regia effectively dissolves gold, forming chloroauric acid. This solution is then treated with additional nitric acid. The nitric acid reacts with other metals present, such as silver, precipitating them as insoluble compounds. These impurities can be easily removed, leaving behind a solution concentrated with gold.
The key to successful refining gold with nitric acid lies in controlling the chemical reactions. The concentration of nitric acid, the temperature, and the duration of the reaction must be carefully managed to ensure that impurities are fully removed while preserving the gold.
Advantages of Refining Gold with Nitric Acid
Refining gold with nitric acid offers several significant advantages. One of the primary benefits is its efficiency in separating gold from other metals. Nitric acid selectively reacts with base metals like silver, making it possible to obtain gold of high purity. This selective action simplifies the refining process, especially when dealing with complex ores or alloys.
Additionally, refining gold with nitric acid is a well-established technique with a long history of success. Its effectiveness in producing high-purity gold makes it a reliable choice for both industrial applications and jewelry manufacturing. The method’s proven track record provides confidence in its results and reliability.
Challenges of Refining Gold with Nitric Acid
Despite its advantages, refining gold with nitric acid presents several challenges. The primary concern is the handling of nitric acid, which is a highly corrosive and hazardous chemical. Proper safety measures must be implemented to protect workers and equipment from its harmful effects. This includes using appropriate personal protective equipment (PPE) and ensuring adequate ventilation to manage fumes.
Another challenge is maintaining precise control over the refining process. Variations in nitric acid concentration, temperature, and reaction time can affect the purity of the final gold product. Therefore, careful monitoring and adjustment are essential to achieving optimal results and minimizing losses.
Environmental and Safety Considerations
The process of refining gold with nitric acid also involves environmental and safety considerations. The use of nitric acid generates waste products that need to be managed responsibly to reduce environmental impact. Additionally, the fumes produced during the process can be hazardous if not properly controlled.
To address these concerns, many refining operations are adopting advanced technologies and practices. This includes improved waste treatment methods, recycling techniques, and better safety protocols. Implementing these measures helps to minimize the environmental footprint and ensure the safety of workers involved in the refining process.



Innovations in Refining Gold with Nitric Acid
Recent advancements in refining gold with nitric acid focus on enhancing both efficiency and safety. Innovations include the development of more effective waste management systems and improved methods for neutralizing hazardous by-products. These advancements aim to make the process more sustainable while maintaining its effectiveness in producing high-purity gold.
Refining gold with nitric acid remains a critical method for achieving high-purity gold. Its ability to selectively remove impurities and produce a concentrated gold solution makes it a valuable technique in the refining industry. While the process involves managing hazardous chemicals and addressing environmental concerns, its benefits in efficiency and reliability make it a preferred choice for many refiners. As technology continues to evolve, the process of refining gold with nitric acid will likely see further improvements, enhancing its sustainability and effectiveness.