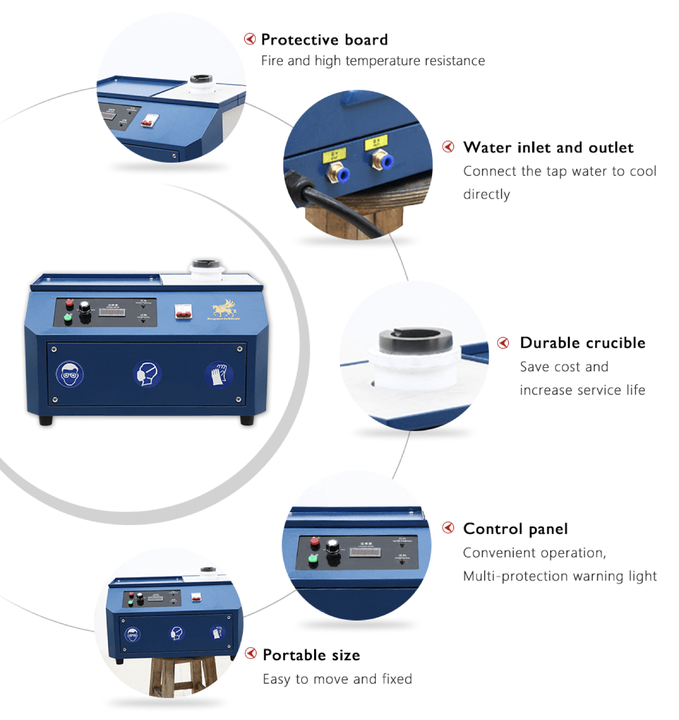
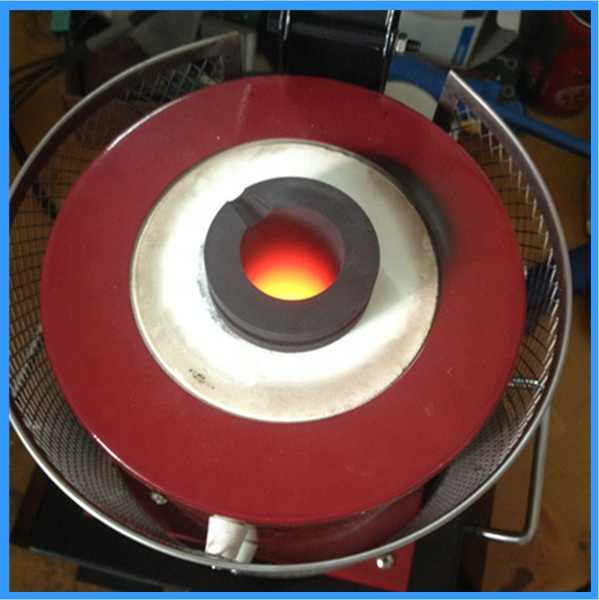
smelting silver chloride
Smelting Silver Chloride Unlocking Pure Silver from Compounds
Silver chloride is a significant byproduct in various refining processes, especially when refining silver ores or reclaiming silver from other materials. While silver chloride is not in its metallic state, it can be smelted to retrieve pure silver. Understanding the smelting silver chloride process is crucial for those involved in metal recovery and refining.
The Basics of Smelting Silver Chloride
Smelting silver chloride involves converting the compound into metallic silver by using heat and chemical reactions. Silver chloride (AgCl) is a compound that forms when silver comes into contact with chlorine or chloride compounds. Although silver chloride is valuable, it is not directly usable as pure silver, and smelting is required to extract the metal in its pure form.
The process typically involves reducing the silver chloride using a reducing agent, such as carbon or iron, to strip away the chlorine, leaving behind metallic silver. Smelting silver chloride requires careful handling, as the chemicals involved can pose environmental and safety concerns.
Why Smelting Silver Chloride is Important
Smelting silver chloride is an essential step in many refining processes. Silver chloride is a common byproduct in processes such as refining gold and silver or recovering silver from scrap materials. Without smelting, the silver remains locked in a compound and cannot be used in its pure metallic form.
By smelting silver chloride, refiners can recover the pure silver, increasing the yield of precious metals from a variety of sources. This process is especially important in industries where high-purity silver is needed for products like jewelry, electronics, and coins.





The Smelting Silver Chloride Process
Preparing Silver Chloride for Smelting
Before the actual smelting process begins, silver chloride must be carefully prepared. The silver chloride is usually washed and dried to remove impurities. This ensures that the smelting process will be more efficient and that the end product will be of higher purity.
Once prepared, the silver chloride is mixed with a reducing agent such as carbon or sodium carbonate. This agent is essential for breaking the bond between silver and chlorine during the smelting process.
Heating and Chemical Reactions
The next step in smelting silver chloride is heating the mixture in a furnace or crucible. High temperatures are required to initiate the chemical reactions that separate silver from chlorine. During this stage, the reducing agent reacts with the chlorine in the silver chloride, forming byproducts such as carbon dioxide or sodium chloride, depending on the agent used.
As the reaction takes place, the silver becomes a liquid metal and settles at the bottom of the furnace or crucible. Meanwhile, the byproducts, such as gas or slag, are removed or left to float on top, allowing the silver to be easily separated.
Recovering the Metallic Silver
After the smelting process, the liquid silver is poured into molds or allowed to cool into solid form. Once solidified, the silver can be removed from the mold and further refined if necessary. At this point, the silver is typically over 99% pure, but additional refining processes can be used to achieve even higher purity levels.
The smelting silver chloride process ensures that the recovered silver is free from the chlorine compound and other impurities, making it suitable for use in various applications.
Challenges in Smelting Silver Chloride
Handling Toxic Byproducts
One of the main challenges in smelting silver chloride is managing the byproducts. Chlorine gas, which can be released during the smelting process, is highly toxic and must be handled carefully. Additionally, depending on the reducing agent used, other harmful gases or slag may be produced, requiring proper ventilation and disposal methods to ensure safety.
High Temperature Requirements
Smelting silver chloride also requires extremely high temperatures, which can pose challenges in terms of energy consumption and the equipment needed. The process must be carefully controlled to maintain the correct temperatures and avoid damaging the furnace or other equipment.
Alternatives to Traditional Smelting Methods
Electrolytic Reduction
For those seeking alternatives to traditional smelting silver chloride, electrolytic reduction offers a different approach. This process uses electricity to reduce silver chloride to metallic silver without the need for high temperatures or reducing agents. While this method can be more energy-efficient and environmentally friendly, it is typically used on a smaller scale compared to traditional smelting.
Chemical Reduction Methods
Chemical reduction is another alternative to smelting, where a reducing agent like sodium hydroxide or sodium carbonate is used to convert silver chloride into silver powder without the need for extreme heat. This method is often favored in laboratory settings or small-scale refining operations where smelting equipment may not be readily available.
While these alternatives have their own benefits, smelting silver chloride remains one of the most widely used and efficient methods for large-scale silver recovery.
Applications of Smelted Silver
Once silver chloride is smelted, the resulting pure silver can be used in a wide variety of applications. Refined silver is highly sought after in industries that require high-quality metal for products such as:
- Jewelry and Silverware: Silver’s luster and durability make it a popular choice for crafting beautiful and long-lasting items.
- Industrial Components: Silver’s high conductivity makes it essential for electrical applications, including switches, conductors, and semiconductors.
- Coins and Bullion: Pure silver is a popular investment, often minted into coins and bars for storage and trading.
By smelting silver chloride, refiners unlock the value of this metal and ensure its availability for diverse applications.
Environmental Considerations in Smelting Silver Chloride
The smelting silver chloride process can have environmental impacts if not managed properly. The release of chlorine gas and other byproducts can harm the environment and pose risks to human health. Therefore, proper ventilation systems and waste management procedures are crucial for minimizing the environmental footprint of silver smelting operations.
Some refiners are also exploring more sustainable methods for silver recovery, such as recycling silver from industrial waste and using cleaner reducing agents that produce fewer harmful byproducts. These efforts are part of a broader movement to reduce the environmental impact of metal refining.
Smelting silver chloride is an essential process in the refining of silver, helping to recover pure silver from its compound form. Through careful preparation, heating, and chemical reactions, silver chloride can be transformed into a valuable metal used across multiple industries. While the process presents some challenges, including handling toxic byproducts and maintaining high temperatures, it remains one of the most effective methods for large-scale silver recovery.