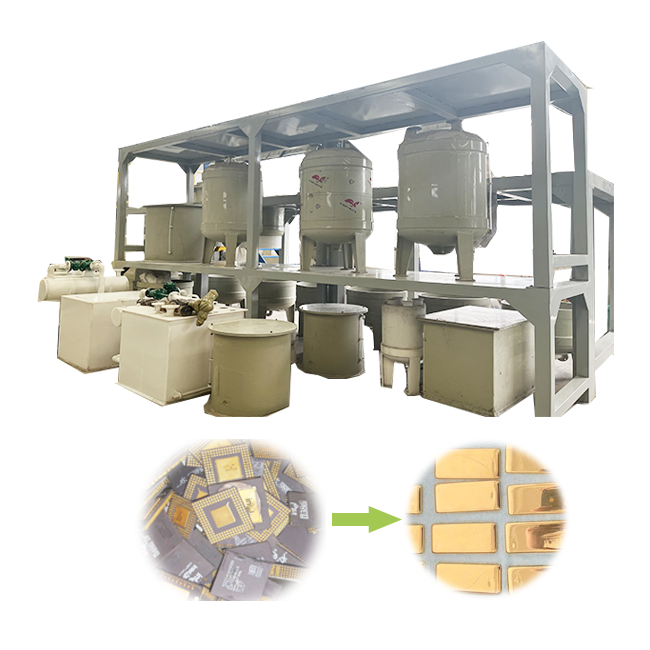
Refining Gold Ore With Nitric Acid
Refining Gold Ore With Nitric Acid: A Comprehensive Guide
Gold refining is a critical process that ensures the purity of gold, making it suitable for various applications from jewelry to electronics. Among several methods used for gold purification, nitric acid plays a pivotal role due to its ability to dissolve base metals effectively while leaving gold relatively unaffected. This article will delve into the specifics of using nitric acid in refining gold ore, highlighting its advantages, processes, and safety precautions.
Nitric Acid’s Role in Gold Refining

Nitric acid (HNO3) is a strong oxidizing agent known for its ability to dissolve many metals except noble metals like gold and platinum. In the context of gold refining, nitric acid serves primarily to remove impurities such as copper, lead, and silver, which are often present in raw gold ore. By selectively dissolving these base metals, nitric acid helps concentrate the gold content, simplifying further purification steps.
Preparation of Gold Ore Before Nitric Acid Treatment
Before applying nitric acid, the gold ore must be prepared properly. This typically involves crushing and grinding the ore to reduce its size, increasing the surface area for better reagent contact. FRT Machinery offers specialized equipment designed for efficient ore reduction, ensuring uniform particle sizes that enhance the effectiveness of subsequent nitric acid treatment.
The Process of Using Nitric Acid in Gold Refining

The process begins by adding the crushed gold ore to a solution of nitric acid. The acid reacts with the base metals, converting them into soluble salts. These salts can then be filtered out, leaving behind a concentrate rich in gold. For optimal results, precise control over factors like acid concentration, temperature, and reaction time is essential. FRT Machinery’s advanced systems provide the necessary precision for this delicate process.
Advantages of Using Nitric Acid in Gold Refining

Using nitric acid for gold refining offers several advantages over traditional methods. It is more environmentally friendly compared to mercury amalgamation, does not require high temperatures like smelting, and is cost-effective for small-scale operations. Additionally, the purity levels achieved through nitric acid treatment are significantly higher, making it a preferred choice among refiners.
Safety Precautions When Handling Nitric Acid
Despite its benefits, nitric acid is highly corrosive and must be handled with extreme caution. Protective gear including gloves, goggles, and respirators should always be worn. Work areas need to be well-ventilated to avoid inhalation of toxic fumes. FRT Machinery emphasizes safety protocols and provides training on safe handling practices to ensure operators’ safety during gold refining processes involving nitric acid.

Innovations in Nitric Acid-Based Gold Refining
Continuous research and development in the field of gold refining have led to innovations aimed at improving efficiency and sustainability. New techniques are being explored that combine nitric acid treatments with other processes for enhanced results. FRT Machinery remains at the forefront of these advancements, developing cutting-edge solutions that push the boundaries of what’s possible in gold purification.
In conclusion, nitric acid plays a vital role in modern gold refining, offering a powerful yet controlled method for removing impurities from gold ore. As technology evolves, so too do the methods and tools available for refining gold, ensuring that this precious metal continues to meet the high standards required across industries.